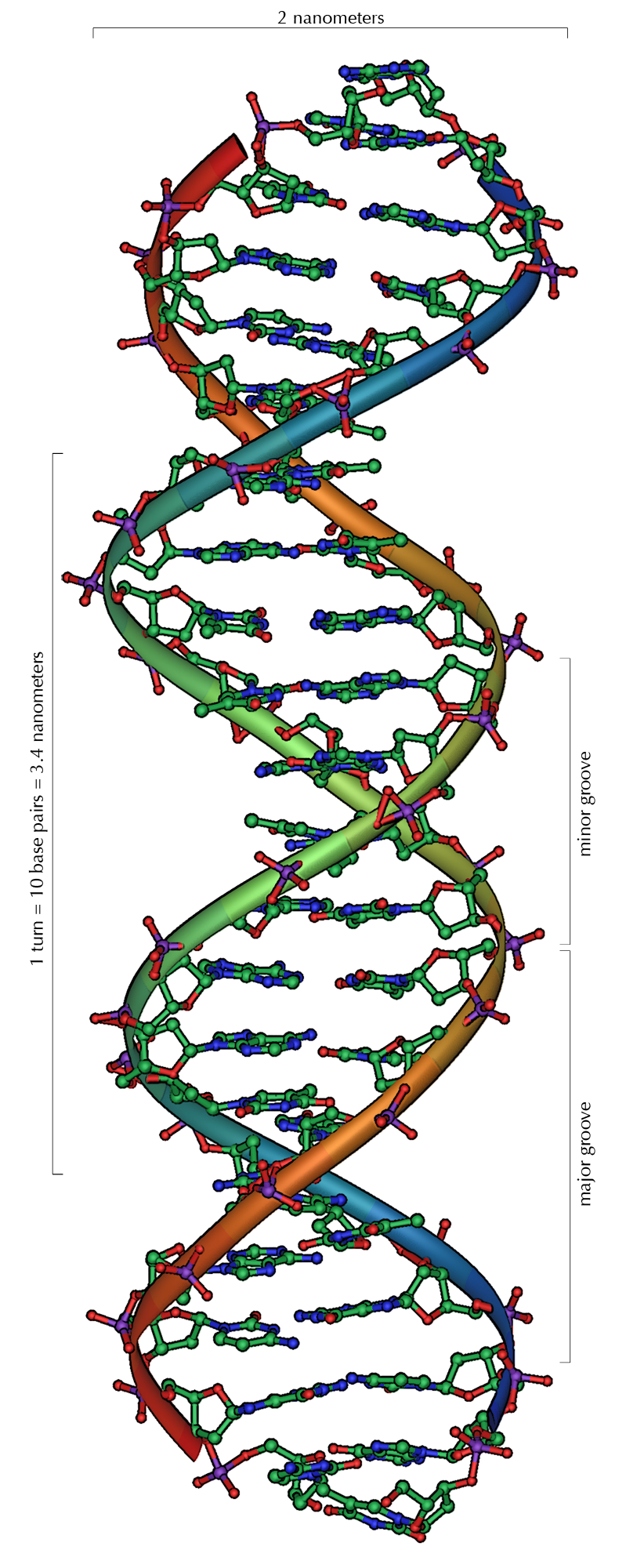
In this unit we focused on the 4 macromolecules. The 4 macromolecules are carbohydrates, lipids, proteins, and nucleic acid. Carbohydrates are sugars that are made with rings of carbon, hydrogen, and oxygen. They are a main source of energy, and come as mono, di, and polysaccharides. Lipids are long chains of carbon and hydrogen, called fatty acids. Lipids are used to store energy, make up cell walls, and produce hormones. There are 2 types of lipids, saturated, which is not healthy for you, and unsaturated which is better for your health. Next we have proteins, which are made up of smaller molecules called amino acids. Proteins support the body, speed up chemical reactions, help cells communicate, and let things through the cell membrane. Lastly, we learned about nucleic acids, which are composed of thousands of repeating nucleotides. Nucleotides are made of phisphate, sugar, and nitrogen. We can see nucleic acids in DNA and RNA.
In addition to the four macromolecules, we learned about ATP and enzymes. ATP is a nucleic acid that is the primary energy transferring molecule in the cell, and enzymes are parts of proteins that speed up chemical reactions. The substrate (molecule enzyme works on) attaches itself to the enzyme on the active site (where the substrate and enzyme are attached. The enzyme then speeds up the reaction and creates the product at a faster rate. Enzymes are important because they can make more product in less time, and lower the amount of energy needed to make a reaction occur.
After learning about these concepts, we did some labs to demonstrate them. The first lab we did was the sweetness lab, where we tasted different sugars to see the difference between the taste of mono, di, and polysaccharides. We found that monosaccharides were generally the sweetest and poly generally the most bland. We also did the cheese lab where we curdled milk to see which conditions are better to curdle milk, and what curdling agent. We learned that the enzyme chymosin in acidic and hot conditions was the best to curdle milk.
All in all, Unit 2 was very informative and taught us a lot in detail about the four macromolecules. The labs were fun, especially the cheese lab because it was very hands on and gave us a good demonstration, opposed to just learning from the textbook. Unit 2 was very fun to learn about, and I hope we can go in more depth over the course of the year.

